

Now, one of the most common types of ionic bonding in nature links silicon, which is element 14, and oxygen, element 8. Quartz, the Most Common Type of Ionic Bonding Learn more about phase transformations and chemical reactions. And that’s because potassium, in the periodic table, in the first column, bonds with iodine, the halogen, in the next to the last column. Because in addition to having sodium chloride, table salt typically has about 1/100th of a percent of potassium iodine, another one of these alkali halides. If we don’t get it other ways, we can get it from table salt. That’s because we need a small amount of iodine in our diet to help the thyroid gland. For example, when you buy salt, you’ll often notice that it’s called iodized salt. Sodium chloride is the most common example of these, but there are many others. (Image: Jiri Hera/Shutterstock)Īnd there are many examples from the periodic table. Salt is formed when chlorine and sodium combine in an ionic bond. Now, because these bonds are between two ions, it is called the ionic bond. The positive and negative ions attract each other, and so you get the bond. Whenever you have an alkali metal, like sodium, that meets up with a halogen, like fluorine or chlorine, they form a salt. Indeed, we see this type of ionic bond in many kinds of common materials, for example, sodium chloride or table salt.
#Ion bonding video series#
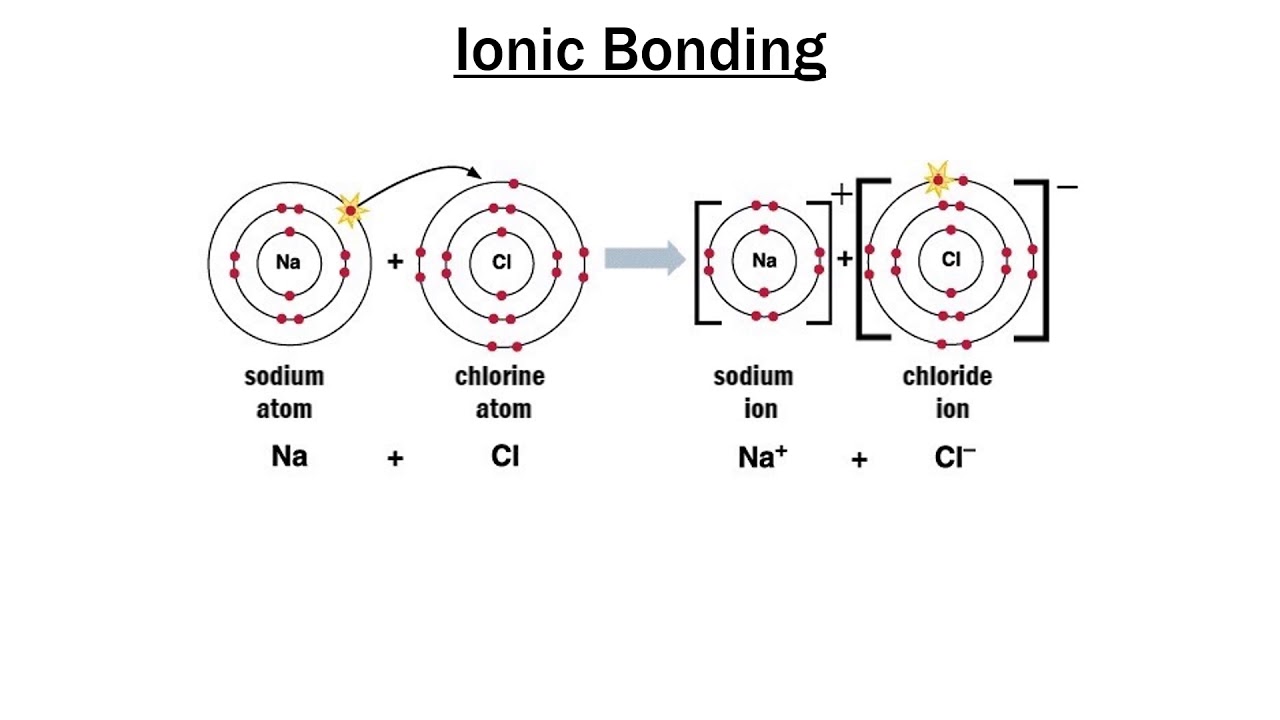
This is a transcript from the video series The Joy of Science. You have a +1 ion, a -1 ion, they see each other and they say, “Ah-ha, electrostatic attraction,” and they bond. And the chlorine, it has 17 positive charges in its nucleus, but now it has 18 electrons, so it’s a -1 ion. When sodium gives up that electron, it now has 11 positive charges in its nucleus, but only 10 electrons, so it’s a +1 ion. And something else happens in the process. I don’t want it.” And so, the sodium’s happy it has 10 chlorine’s happy, it has 18. Well, naturally the sodium says, “Here, take my electron. So, imagine what happens when a chlorine atom meets a sodium atom. By the same token, sodium, which is element 11, has one too many electrons, and it’s going to do almost anything it can to lose an electron. It’ll do almost anything it can to gain that electron. Chlorine, element 17, for example, has one too few electrons. (Image: Gravity Digital/Shutterstock) Formation of Ionic Bonding A ceramic mug is an example of ionic bonding. In such an event, the world of chemistry would be boring. Imagine what would happen if all combinations of electrons had exactly the same energy.
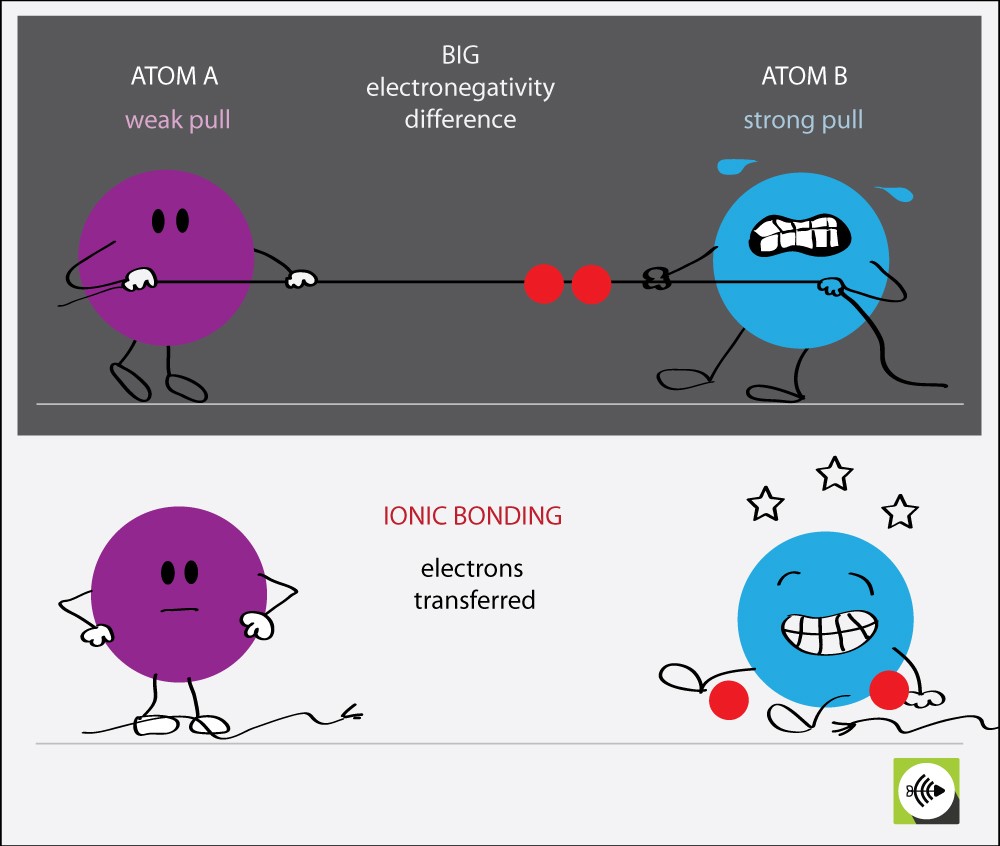
, George Mason University In thinking about ionic bonding, the key is to remember that atoms or molecules with magic numbers of electrons are particularly stable, while atoms or molecules that are one or two electrons too many or too few are unstable.


 0 kommentar(er)
0 kommentar(er)
